2018 Activity Report for Mission 5-2: Establishing a Society with Reduced Dependence on Fossil Resources: Plants, Biomass, Energy, and Materials
Updated: 2019/04/25
Research 1: Tailor-made breeding of grass biomass through lignin bioengineering
RISH investigator(s): Toshiaki Umezawa, Yuki Tobimatsu, Shiro Suzuki
Outside collaborator(s): Tokushima University, Nara Institute of Science and Technology (NAIST), Earthnote Co., The University of Hong Kong, University of Wisconsin, US DOE Bioenergy Research Center, CAS Shanghai Institute of Plant Physiology and Ecology, etc.
To explore new breeding strategies to improve the production of bioenergy and biomaterials from grass biomass, this project seeks to develop and characterize transgenic rice plants that produce biomass with variously modified lignin contents and structures. To this end, new lignin-modified transgenic rice lines are being developed via up- and/or down-regulations of lignin biosynthesis pathway genes. We are also working on the selection and breeding of grass biomass crop varieties that show superior lignin characteristics.
 |
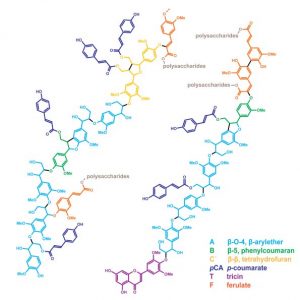 |
Figure: Lignin-modified transgenic rice plants grown in a greenhouse (left) and structural models of lignins in grass biomass or cell walls (right).
Publications, etc.
- Miyamoto, T. et al., OsMYB108 loss-of-function enriches p-coumaroylated and tricin lignin units in rice cell walls. The Plant Journal, in press, doi:10.1111/tpj.14290, 2019.
- Lam, P. Y. et al., Recruitment of specific flavonoid B-ring hydroxylases for two independent biosynthesis pathways of flavone-derived metabolites in grasses. New Phytologist, in press, doi:10.1111/nph.15795, 2019.
- Mutuku, J. M. et al., The structural integrity of lignin is crucial for resistance against Striga hermonthica parasitism in rice. Plant Physiology, in press, doi:10.1104/pp.18.01133, 2019.
- Takeda, Y. et al., Lignin characterization of rice CONIFERALDEHYDE 5‐HYDROXYLASE loss‐of‐function mutants generated with the CRISPR/Cas9 system. The Plant Journal, 97, 543-554, doi:10.1111/tpj.14141, 2019.
- Takeda, Y. et al., Comparative evaluations of lignocellulose reactivity and usability in transgenic rice plants with altered lignin composition. Journal of Wood Science, 65, 6, doi:10.1186/s10086-019-1784-6, 2019.
- Tobimatsu, Y. and Schuetz, M. Lignin polymerization: how do plants manage the chemistry so well? Current Opinion in Biotechnology, 56, 75-81, doi:10.1016/j.copbio.2018.10.001, 2019.
- Takeda, Y. et al., Downregulation of p‐COUMAROYL ESTER 3‐HYDROXYLASEin rice leads to altered cell wall structures and improves biomass saccharification. The Plant Journal, 95, 796-811, doi:10.1111/tpj.14141, 2018.
- Miyamoto, T. et al., A comparative study of the biomass properties of Erianthus and sugarcane: lignocellulose structure, alkaline delignification rate, and enzymatic saccharification efficiency. Bioscience, Biotechnology and Biochemistry, 82, 1143-1152, doi:10.1080/09168451.2018.1447358, 2018.
- Miyamoto, T. et al., Comparative analysis of lignin chemical structures of sugarcane bagasse pretreated by alkaline, hydrothermal, and dilute sulfuric acid methods. Industrial Crops and Products, 121, 124-131, doi:10.1016/j.indcrop.2018.04.077, 2018.
- Umezawa, T. Lignin modification in planta for valorization. Phytochemisty Reviews, 17, 1305-1327, doi: 1007/s11101-017-9545-x, 2018.
Research 2: Metabolite production platform using lipid-secreting plant cells
RISH investigator(s): Kazufumi Yazaki, Akifumi Sugiyama
Outside collaborator(s): RIKEN
Plants have developed lipid secretion mechanisms to protect themselves from dryness since the land colonization in ancient ages. This lipid secretion ability is especially prominent in epidermal cells, where wax and cutins as well as suberin are secreted. In term, some other characteristic cell types related to oil glands and glandular trichomes are also involved in secretion of lipid-soluble low molecular weight substances, like secondary metabolites. One of such examples is Lithospermum erhthrorhizon, in which root epidermal cells are secreting a large amount of secondary metabolic lipids, i.e., shikonin derivatives. The cultured cells of this plant show shikonin productivity of ca. 10% of cell dry weight. In this study, we are trying to develop a platform to produce high value lipid-soluble metabolites utilizing the lipid secreting ability of this plant cells. In the fiscal year 2018, we have conducted multiple omics studies i. e., transcriptome and proteome analyses in cultured L. erythrorhizon cells, hairy root cultures and the intact roots of this plant, to make a list of genes potentially relevant for the secretion of secondary metabolic lipids, shikonin derivatives. A strong advantage of this plant system is that shikonin production is restricted in darkness and epidermal cells, by which clear negative controls can be obtained (light irradiation, or pealed root tissues). While candidates picked up from transcriptomics and proteomics did not largely overlapped, we have selected top 16 candidate genes, which appeared to correlate with the production and secretion shikonin derivatives.
2-300x215.png) Figure: Metabolite production platform using lipid-secreting plant cells
Figure: Metabolite production platform using lipid-secreting plant cells
Publications, etc.
- Takanashi et al., Comparative proteomic analysis of Lithospermum erythrorhizon reveals regulation of a variety of metabolic enzymes leading to comprehensive understanding of the shikonin biosynthetic pathway. Plant Cell and Physiology, 60, 19-28, doi:10.1093/pcp/pcy183, 2019.
- Kitajima, S. et al., Comparative multi-omics analysis reveals diverse latex-based defense strategies against pests among latex-producing organs of the fig tree (Ficus carica), Planta, 247, 1423-1438, doi:10.1007/s00425-018-2880-3, 2018.
- Kusano, H. et al., Establishment of a modified CRISPR/Cas9 system with increased mutagenesis frequency using the translational enhancer dMac3 and multiple guide RNAs in potato, Scientific Reports, 8, 13753, doi: 1038/s41598-018-32049-2, 2018.
Research 3: Conversion of biomass into chemical resources using a microwave and biological process
RISH investigator(s): Takashi Watanabe, Hiroshi Nishimura
Outside collaborator(s): Nippon Kayaku Co., Taiyo Nippon Sanso Co., Kyoto University ICR, Kyoto University IAE, Tottori University, Thailand National Science and Technology Development Agency (NSTDA), Chulalongkorn University, Indonesian Institute of Science (LIPI), etc.
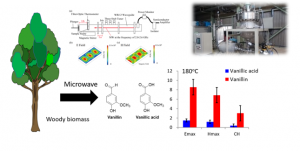
We are studying conversion system of tropical and domestic biomass into functional chemicals and biofuels. In FY 2018, we studied biorefinery system of sugarcane trash including functional analysis of cellulases from metagenome, microwave pretreatments and production of surfactants from residual lignin, together with National Science and Technology Development Agency (NSTDA), Thailand and Indonesian Institute of Sciences (LIPI). The collaborative research was accepted as a new e-Asia program, and starts as a multilateral cooperative project of 4 countries including Laos National University from FY2019. We published research articles including microwave pretreatments using a new Lewis acid catalysts.
 |
 |
 |
Figure: Continuous 915 MHz microwave reactor developed by CREST project and experiments of wood degradation by a copper oxide reaction which produces vanillin in 2-3 times higher yields than those of conventional heating. Experiments using a cavity resonator revealed that electric field significantly accelerated the reaction. A thermostable polymer was synthesized from vanillin produced by the microwave reaction of Japanese cedar wood.
 |
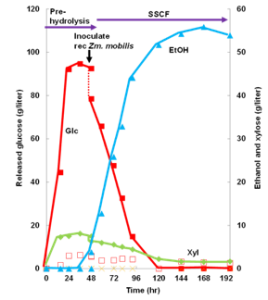 |
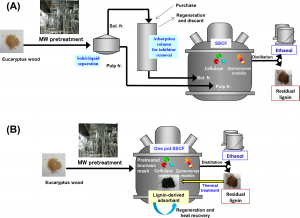
Figure: Self-sufficient bioethanol production system was developed using a residual lignin-derived adsorbent for fermentation inhibitors. The adsorbent was prepared by thermal treatment of the lignin released in a process of bioethanol production.
Publications, etc.
- Ohashi, Y. and Watanabe, T. Catalytic performance of food additives alum, flocculating agent, Al(SO4)3, AlCl3 and other Lewis acids in microwave solvolysis of hardwoods and recalcitrant softwood for biorefinery, ACS Omega, 3, 16271–16280, doi: 1021/acsomega.8b01454, 2018.
- Isozaki, K. et al., Robust surface plasmon resonance chips for repetitive and accurate analysis of lignin–peptide interactions, ACS Omega, 3, 7483-7493, doi:10.1021/acsomega.8b01161, 2018.
- Press release, http://www.jst.go.jp/pr/info/info1357/besshi1.html; http://www.jst.go.jp/pr/info/info1357/Appendix1. html.
- Tokunaga, Y. et al., NMR analysis on molecular interaction of lignin with amino acid residues of carbohydrate-binding module from Trichoderma reesei Cel7A, Scientific Reports, 9, 1977, doi:10.1038/s41598-018-38410-9, 2019.
- Press release, http://www.kyoto-u.ac.jp/ja/research/research_results/2018/190213_1.html
Research 4: Design of green biomass conversion based on an analysis of branched structures on lignocellulose
RISH investigator(s): Hiroshi Nishimura, Takashi Watanabe
Outside collaborator(s): Chalmers University of Technology, Wallenberg Wood Science Center (WWSC), Kyoto University ICR, etc.
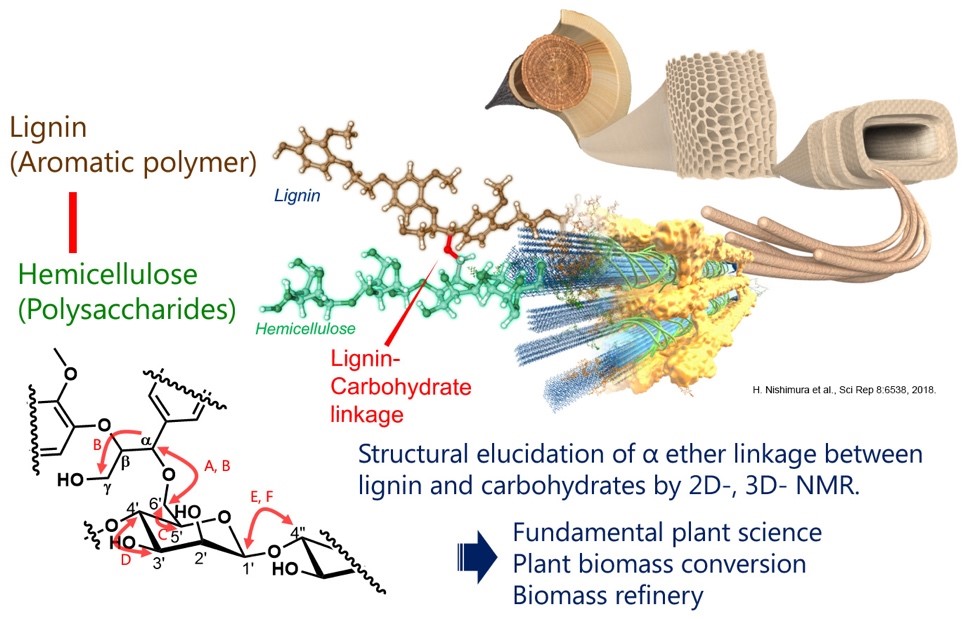
Advanced utilization of plant biomass needs a deeper understanding of the molecular structure of lignocellulose polymers. In particular, branched structures on lignocellulose, e.g., lignin-polysaccharide linkages, are critical to elucidate for converting biomass to useful chemicals, materials, and energy. Here, we established a highly concentrated fraction of lignin-carbohydrate complexes and purified by combining of carbohydrase enzymatic treatment and sequential chromatography. We succeeded in proving the covalent bond between lignin and hemicellulose by two-dimensional and three-dimensional NMR method. We are now trying to develop the environment-friendly green system of biomass conversion based on the accurate molecular structure analysis.
Figure: Study overview of the lignin-carbohydrate linkage elucidation
Publications, etc.
- Nishimura, Y. et al., Direct evidence for α ether linkage between lignin and carbohydrates in wood cell walls, Scientific Reports 8, 6538, doi:10.1038/s41598-018-24328-9, 2018.
- Nishimura, H., The elucidation of branched structures in lignocellulose — Multi-dimensional NMR analyses for the linkages between lignin and polysaccharide, AgriBio, 2, 9, 64-66, 2018. (in Japanese)
- Press release: http://www.kyoto-u.ac.jp/ja/research/research_results/2018/180425_5.htm.
Research 5: Development of high-strength products based on cellulose and chitin nanofibers
RISH investigators: Hiroyuki Yano, Kentaro Abe
Outside collaborator(s): Chuchu Chen (Nanjing Forestry University)
Recently, natural nanofibers such as those of cellulose and chitin, which are major components of plant cells and the shells of crabs and shrimp, respectively, have received great interest by virtue of their outstanding mechanical properties. The aim of this study is to develop high-strength products (film, spun fiber, filters, etc.) based on cellulose and chitin nanofibers. Recently, we provided an alternative approach for the preparation of quinone- crosslinked Chitin nanofiber-based hydrogels using amino groups, which inspired by the quinone hardening process during insect cuticle sclerotization. Our recent studies have been reported in the following papers. Figure. Wet-spun fiber based on cellulose nanofiber.
Figure. Wet-spun fiber based on cellulose nanofiber.
Publications, etc.
- Chen, C. et al., Formation of high strength double-network gels from cellulose nanofiber/polyacrylamide via NaOH gelation treatment. Cellulose, 25, 5089-5097, 10.1007/s10570-018-1938-5, 2018.
- Yang X. et al., Extremely stiff and strong nanocomposite hydrogels with stretchable cellulose nanofiber/poly(vinyl alcohol) networks. Cellulose, 25, 6571-6580, doi:10.1007/s10570-018-2030-x, 2018.
- Abe, K., Novel fabrication of high-modulus cellulose-based films by nanofibrillation under alkaline condition. Carbohydrate Polymers, 205, 488-491, doi:10.1016/j.carbpol.2018.10.069, 2018.
- Chen, C. et al., Bioinspired hydrogels: quinone crosslinking reaction for chitin nanofibers with enhanced mechanical strength via surface deacetylation. Carbohydrate Polymers, 207, 411-417, doi:10.1016/j.carbpol.2018.12.007, 2019.
Research 6: Development of energy storage device from biomass
RISH investigator(s): Toshimitsu Hata
Research collaborator(s): Lignyte Inc., Indonesian Institute of Science (LIPI), Osaka Prefecture University, etc.
Development of energy storage device from biomass is promising due to the view of renewability, low cost, and sustainability. In this study, we prepared composite precursor from phenol resin and cellulose nanofiber (CNF). CNF and 50% KOH solution was added to phenol resin, and then carbonized/activated. Specific surface area and electrochemical property of carbonized carbon materials was evaluated with gas measurement and charge-discharge behavior, respectively. The discharge capacity of the capacitor from activated composite was higher than that of activated phenolic resin. The easiness of ion diffusion in the obtained 3D carbon structure may contribute to the discharge capacity.
Publications, etc.
- Onishi, Y., Hata, T.et al, Development of electric double layer capacitor using cellulose nanofiber composite solid phenol resin as electrode, The 16th annual meeting of wood carbonization society (2018 June).
- Onishi, Y., Hata, T.et al, Characteristics of electric double layer capacitor of composite of phenolic resin and cellulose nanofiber, The 45th (2018 Dec).
- Hata, et. al. Development of energy storage device from biomass, 6th JASTIP Symposium, Tangerang, Indonesia (2018 Nov).
Research 7: Development of IoT technology with wireless power transfer via microwaves
RISH investigator(s): Naoki Shinohara, Tomohiko Mitani
Research collaborator(s): Mitsubishi Heavy Industries Ltd., Panasonic Co., Sho Engineering Co., etc.
Toward a post-fossil-fuel society, IoT (Internet Of Things) technology for a sophisticated society is required. In this project, we are developing an unconscious wireless charging system and battery-less sensor with wireless power transfer via microwaves. We propose new IoT technology with wireless communication and wireless power and carry out field experiments. In 2018, we simulated new rectenna (rectifying antenna), whose characteristics was not changed by bending or/and on-body application, for wearable battery-less sensor.
-2-300x95.png)
Fig. Image of Battery-Less Wearable Sensor, New Bent Rectenna Simulated in 2018, and Its Characteristics
Publications, etc.
- Yang, B. et al., Evaluation of the modulation performance of injection-locked continuous-wave magnetrons, IEEE-Trans. ED, 65, 1-7, doi: 1109/TED.2018.2877204, 2018.
- Tanaka Y. et al., Development of low power wireless sensor powered by microwave wireless power transfer (in Japanese), IEICE-Trans. B, J101-B, 968-977, doi:10.14923/transcomj.2018EEP0008,
- Press release: BBC Arabic “BBC News 4Tech مشروع لنقل الطاقة الكهربائية لاسلكياً” (2018, Sep. 5).
Research 8: Estimation of microwave impacts on insect ecology from human activity
RISH investigator(s): Aya Yanagawa, Tomohiko Mitani
Research collaborator(s): INRA, Nara University of Education, Tezukayama Senior High School, Kyoto Institute of Technology.
The demand for microwave technology will continue to increase. Recently numbers of problems from the human activities show up such as the issues about microplastic contamination, honey bee colony collapsing, climate change etc. The possibility of serious impacts has been known but happened unexpectedly. Microwave is known to have an impact on living organism and its usage is carefully handled for human safety. While the influence on the other living organisms is not counted yet. For the safety and development of the IoT (Internet of Things), it is essential to learn its impacts on nature. We would like to establish a method for estimating such impact by using insect ecology as a model system.
Publication
- Yanagawa, A., If insect sense electromagnetic field? HSS2018/8th ISSH, Medan, Indonesia (2018 Nov).


-1-300x154.png)
-300x153.png)
